In this chapter, we will elaborate on the factors that need to be focussed on while conducting a feasibility analysis.
One of the key processes that occurs in parallel to designing a clinical study is evaluating the feasibility of conducting the study in a particular country of interest. However, since the smooth running of a clinical trial depends on timely availability of appropriate logistics and infrastructure, a thorough feasibility analysis is critical to the clinical trial process. In this chapter, we will elaborate on the factors that need to be focussed on while conducting a feasibility analysis.
So what are some of the issues that need to be considered at the start of feasibility evaluation?
Depending on the therapeutic indication, these are prevalence data, market research, and available infrastructure and facilities in the country where the trial is planned to be conducted. This involves not only gathering country data but also conducting in-depth interviews with key opinion leaders, medical professionals and specialists experienced in clinical investigations, in order to collect information on contemporary guidelines and practices and standards of care in the country. This process allows the sponsor to get a clinical perspective as well as practicalities and limitations if the country is to be shortlisted as a study site.
Once a country has been chosen for conducting clinical trials in, the next most important part is identifying the best research centres to implement these. In most cases, this task will be run concomitantly to identifying the target country. Factors that influence selection of study centres include specialisation in the indication, availability of investigators at the centre, clinical research expertise of the centre, availability of the necessary diagnostic and interventional infrastructure at the centre and sources of subject recruitment.
Having covered what essentially comprises the feasibility evaluation process, it is then germane to shift the discussion to practical questions such as “What constitutes a good centre?” or “What special considerations do we need to take into account during feasibility analysis?” Given our experience with numerous trials over years, we have identified certain factors as essential for the conduct of clinical studies.
One of the critical factors essential for successfully running clinical trials is proper documentation and reporting of the research process. Ensuring methodical collection and storage of trial data including source records, and maintaining these in line with ALCOAC principles, not only affirms the chronological sequence of the process but also helps trial monitors and non-study personnel such as auditors and inspectors confirm adherence to the study protocol and all regulatory guidelines.
Another key factor is the quality of services provided by the institution. This includes a thorough understanding of the highly regulated environment of clinical trials and consequent requirements, the availability of a well-trained team comprising medical professionals and support staff who operate under GCP guidelines, as well as appropriated, certified facilities enabling proper completion of the study procedures.
Also important is prompt and timely communication. This could be between the institution and the sponsor or their representatives, e.g. in terms of collection of documents required for the completion of the study application, submission of these to the ethics committee and regulatory bodies, as well as in terms of financial contract negotiations and signing.
Yet another issue which has a substantial impact on study timelines is subject recruitment. Studies have shown that almost 80% of clinical trials do not meet enrolment timelines,[1] and half of the sites do not recruit as many subjects as they expect to.[2] Hence, declared recruitment is an issue that needs to be assessed pragmatically considering detailed protocol requirements.
While conducting feasibility analysis our staff evaluates whether the prospective centres satisfy these essential criteria. We ensure that the study centre has documentation systems and back-ups, and other related SOPs in place, prior to signing the agreement. Besides this, we also ensure that the study staff is in place and identify key personnel such as the study investigator, study coordinator(s), study nurse, etc., as well as the hierarchy of responsibility. It is in such practical matters that our experience as a CRO and our awareness of the ground situation and potential pitfalls offers a productive partnership to sponsors wishing to conduct clinical trials. Already at this stage we are able to predict the future quality of cooperation with a particular site.
According to a PricewaterhouseCoopers report published in 2015, there are six key advantages, which make Poland attractive for clinical trial sponsors. They are:
• Large population of patients
• The size and maturity of the market
• Qualified and experienced medical staff working in well-organized and specialist research sites, as well as a strong sponsor/CRO market and qualified and experienced CRAs
• Attractive level of clinical trials costs versus Western Europe or other Central and Eastern European countries. [3]
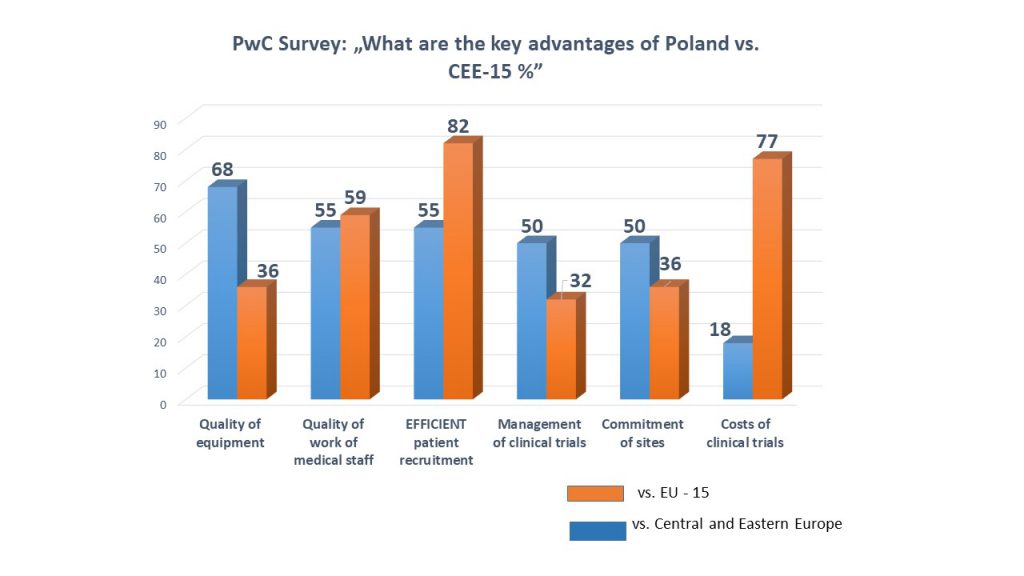
The chart shows the results of the survey conducted by PwC and published in the report mentioned above.
The question posed was: ‘What are the key advantages of Poland versus Central and Eastern Europe and EU-15 countries?’
In the Central and Eastern Europe region Poland has been indicated to be a leader in terms of the quality of medical equipment and staff, as well as high commitment of clinical research sites and good recruitment.
According to the survey, the key competitive advantages of Poland versus the EU-15 (fifteen European Union countries before the last enlargement) are: efficient patient recruitment and lower costs of clinical trials. Respondents also indicated a medical staff work quality. 5
While conducting feasibility analysis our staff evaluates whether the prospective centres satisfy these essential criteria. We ensure that the study centre has documentation systems and back-ups, and other related SOPs in place, prior to signing the agreement. Besides this, we also ensure that the study staff is in place and identify key personnel such as the study investigator, study coordinator(s), study nurse, etc., as well as the hierarchy of responsibility. It is in such practical matters that our experience as a CRO and our awareness of the ground situation and potential pitfalls offers a productive partnership to sponsors wishing to conduct clinical trials. Already at this stage we are able to predict the future quality of cooperation with a particular site, which results in the quality of the study.
In the next topic we will discuss a
feature crucial to successfully conducting clinical trials – patient
recruitment.
[1] Clinical trial delays: America’s patient recruitment dilemma (2012). Available from: http://www.drugdevelopment-technology.com/features/ featureclinical-trial-patient-recruitment/featureclinical-trial- patient-recruitment-1.html
[2] Kaitin KI. 89% of trials meet enrollment, but timelines slip, half of sites under-enroll. Tufts Center for the Study of Drug Development Impact Report. 15(1) (2013). Available from: https://csdd.tufts.edu/s/Jan-Feb-2013-IR-summary.pdf
[3] PricewaterhouseCoopers Group. Clinical trials in Poland. December 2015. Available at: https://www.pwc.pl/pl/pdf/clinical-trials-in-poland-pwc-report.pdf

