Clinical Trials
ACT provides a full range of CRO services and can support you along the whole process of a clinical trial in any therapeutic area.
ACT offers comprehensive assistance in submission process for companies from the EU as well as from outside, wishing to introduce medicinal products and medical devices to the market.
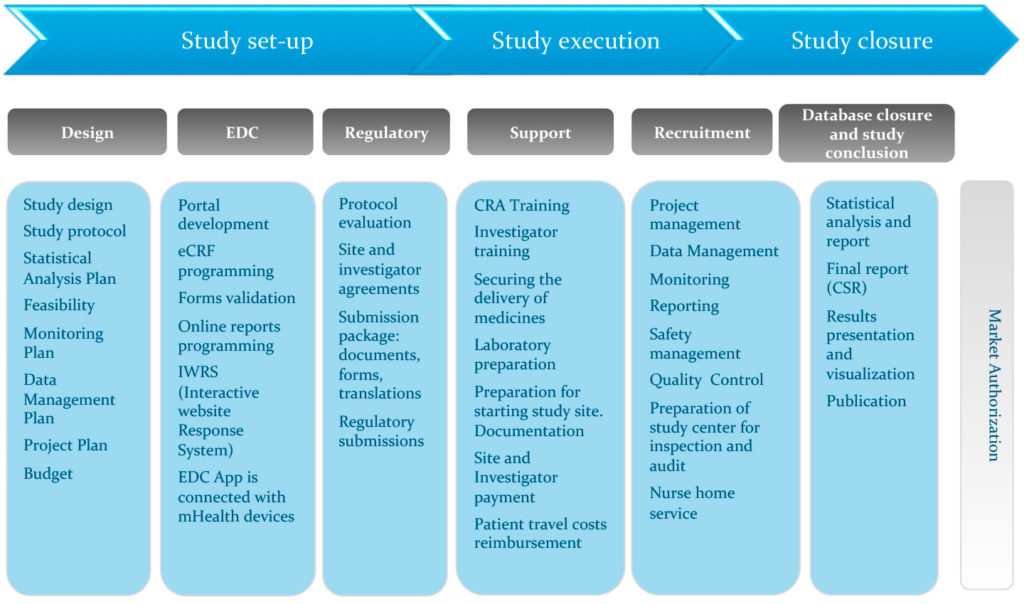
Set up
Design
We know how crucial for project outcome the study design is. That’s why we propose you full project construction in accordance with your expectations. We take care of the protocol development and draw up statistical plan, data management plan and many other essential documents indispensable for the proper implementation, conduct and completion of the study.
Regulatory
ACT provides a wide range of services concerning study set up and submission. We translate all kinds of documents (e.g. patient information and consent, other patient materials, laboratory guidelines, etc.) and adapt them to the Polish law. We take responsibility for collecting appropriate documentation and submitting studies to the relevant authorities, in order to obtain the required approvals without delay. We ensure professional supervision of the whole process as well as efficient communication between the authorities and the sponsor.
Feasibility
ACT offers a comprehensive feasibility study to our Polish and foreign clients. We are able to indicate required clinical sites in a short term. We understand how important is the right choice of an investigative center and qualified personnel, so we pay great attention to the feasibility process as a powerful tool for site and staff suitability verification.
From the beginning of the study, we dedicate our time and knowledge, which always pays off in later stages.
Budget planning
Having years of experience ACT can offer customers support with study budget planning, taking into consideration all aspects of the study including local conditions, constraints and realities.
We closely follow all activities in the study and check the adherence to the planned budget.We indicate the need of budget modification if necessary. We also propose vendors’ contracts and budgets control.
eCRF and data management
We are using cutting-edge tools for CRF electronic data collection and management. Our integrated digital solutions include electronic patient questionnaires (ePROs) or mobile devices that examine patients’ clinical parameters (mHealth). We are able to integrate electronic source documentation (eSource) and when needed, we can combine Real World Data with a randomized clinical trial database.
The use of our electronic tools during the course of the study will:
- ensure compliance with industry requirements and standards (eg FDA CFR21 Part11),
- give instant access to all information, from any place and at any time,
- enable constant supervision over patient safety,
- reduce necessary monitoring visits at sites,
- reduce time to study outcomes evaluation.
Study Execution
Site selection
Considering the feasibility process and our experience, we are able to identify the weak and strong points of potential study sites, suggest the best centers for specified profile of the study and provide a suitable approach for each of the chosen centers. An accurate assessment of the clinical sites is an opportunity to provide the right number of patients required in the clinical trials. We work closely with our centers to identify the appropriate patient population.
We always work with sites and investigators whose quality of services we know from our previous experience or which are recommended by our colleagues, CRO or sponsors.
Contracts
We have a wide experience in preparing and executing contracts with sites, hospital authorities, investigators, other site staff and involved third parties. We take responsibility for all activities and communication, which includes respecting deadlines with regards to signing all documents needed for study submission and start. We are also experienced with handling confidential information, protecting our sensitive data and respecting fair competition law.
Investigators training
In cooperation with our partners in business, we organize local and international meetings, where we provide appropriate training and information to all parties involved in the trial. This includes protocol, Good Clinical Practice (GCP), study of specific procedures, data capture and all other kinds of training according to the sponsor requirements. We also consider an initiation visit as a very important tool for advanced training on the study procedures for all site staff, in order to ensure that the trial is carried out smoothly and efficiently.
Project management
Our experience in project management allows us to keep supervision over any step of the study and to monitor all activities of the parties involved. We pay a lot of attention to using the work time of the study team in the planned activities in the clinical trials. We are eager to cooperate closely with the study sponsor, in order to respect the study timelines and budget.
Study monitoring
ACT personnel consists of well educated professionals with excellent interpersonal skills and high level of responsibility. We pay great attention to regular staff training in order to meet international standards. All personnel involved in the trial receives study-specific training and sponsor SOP training if applicable before the beginning of the project. We ensure the conduct, documentation and reporting of clinical trial in accordance with the protocol, SOPs, Good Clinical Practice and applicable regulations.
Investigators and site staff payments
We supervise the process of investigators and site staff fee payments and provide sites and sponsor with updated information and payments record.
Reimbursement of patient’s travel costs
We provide full management of patient’s travel and accommodation costs reimbursement, according to Polish regulations in force and customer’s requirements.
Clinical trial nursing services
Together with our business partner, we offer nursing support in clinical trials which allows to complete some procedures at the patient’s home. We manage all planning, monitoring and training activities.
We cooperate with a group of 250 nurses in Poland, and over 190 in Hungary, Slovakia and the Czech Republic, trained in GCP procedures. 50% of them have higher nursing education (bachelor’s or master’s degree). 20% speak English.
Thanks to nurses’ home visits patients do not have to travel to and from the clinical center. We also manage transport of collected samples and materials.
So far we offer the nursing service in Poland, Slovakia, the Czech Republic and Hungary. Soon also in Lithuania, Ukraine and Romania.
Audits
We believe that the quality in clinical operations is of the highest importance. That is why we propose our services in conducting sites’ and study audits and other quality-related activities, in accordance with our customer’s needs and worldwide standards.
Logistics services
In cooperation with our partners in business we offer clinical packaging and labelling, qualified transport and destruction of medicinal products in accordance with GMP and GDP.
Study closure
At the end of the study we support our clients with activities related to the study summary and closure.
We take care of the statistical analysis and Clinical Study Report (CSR), as well as of presentation of project results, e.g. publication or product market authorization.
